Percentage of Copper in Brass
MORE LAB RESOURCES 🔒
Student Procedure
Teacher Annotated Procedure
Sample Data, Calculation & Analysis
GoogleSheet Data & Analysis
SUMMARY/ OVERVIEW:
The objective of this lab is to determine the percentage of copper in a brass sample using spectrophotometry. Brass is an alloy composed primarily of copper and zinc. By dissolving the brass sample and analyzing its copper content, students gain insight into quantitative analysis techniques.
ESTIMATE TIME ⏰: 1.5 - 2.5 hours
Preparation of Standard Solutions (20–30 minutes)
Dissolution of Brass Sample (30–45 minutes)
Measurement of Unknown Sample (10–20 minutes)
SAFETY PRECAUTIONS:
Concentrated nitric acid (HNO3) is severely corrosive, a strong oxidizer, and toxic by ingestion and inhalation. Reactions of nitric acid with metals generate nitrogen dioxide (NO2), a toxic , reddish brown gas. Work with nitric acid only in a fume hood.
Copper (II) sulfate and copper (II) nitrate solution are toxic and irritating to skin.
Iron (III)chloride and iron (III) nitrate solutions may be skin and body tissue irritants.
Zinc nitrate solution is toxic and irritating to body tissue. Zinc sulfate is a mild body tissue irritant.
Avoid contact of all chemical with eyes and skin. Wear chemical splash goggles, chemical-resistant gloves, and a chemical resistant apron or lab coat. Please follow all normal laboratory safety guidelines. Wash hands thoroughly with soap and water before leaving the laboratory.
Background Information
Brass is a generic term for alloys of copper and zinc. In addition to these metals, brass may also contain small amounts of iron, lead, aluminum, and tin. More than 300 different brass alloys are known, with uses ranging from decorative hardware to architectural construction, musical instruments, and electrical switches. The amount of copper in brass affects its color, hardness, ductility, mechanical strength, electrical conductivity, corrosion resistance, etc. Increased amounts of zinc provide the material with improved strength and ductility. If the zinc content of the brass ranges from 32% to 39%, it will have increased hot-working abilities, meaning that it can be shaped or bent easier when the metal is heated, but the cold-working will be limited. If the brass contains over 39% zinc, it will have a higher strength and lower ductility (at room temperature).
Watch Copper Reacting with Nitric acid
4 HNO3 (l) + Cu(s) ⟶ Cu(NO3)2 (aq) + 2 NO2 (g) + 2 H2O (l)
The copper nitrate salt that forms is a deep blue/ green color. The nitrogen dioxide is a maroon vapor.
Brass can range in color from red to yellow depending on the amount of zinc added to the alloy. Around 3000 B.C., Ancient metalworkers in the area now known as Syria or eastern Turkey knew how to metal copper with tin to make bronze alloys. Sometimes they made brass without knowing it, because tin and zinc ore deposits are sometimes found together and the tow materials have similar colors and properties. By about 20 B.C., metalworkers around the Mediterranean Sea were able to distinguish zinc ores from those containing tin and began zinc with copper to make brass coins and other items. Most of the zinc was obtained by heating a mineral, calamine, which contains several zinc compounds. Around 300 A.D. , brass metalworking had become established in northern Europe in Germany and the Netherlands.
Experimental Set-up
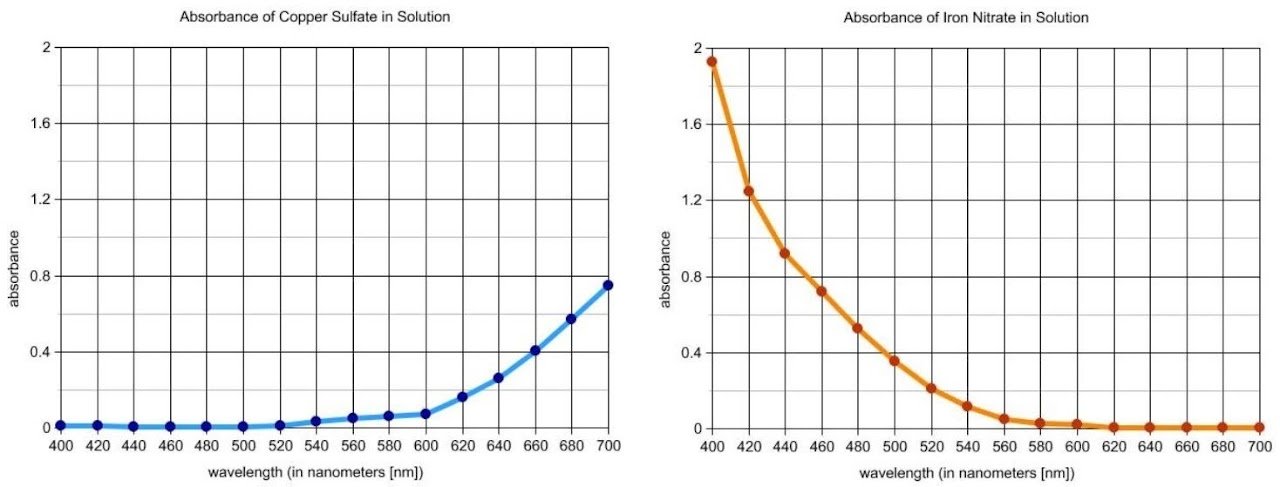
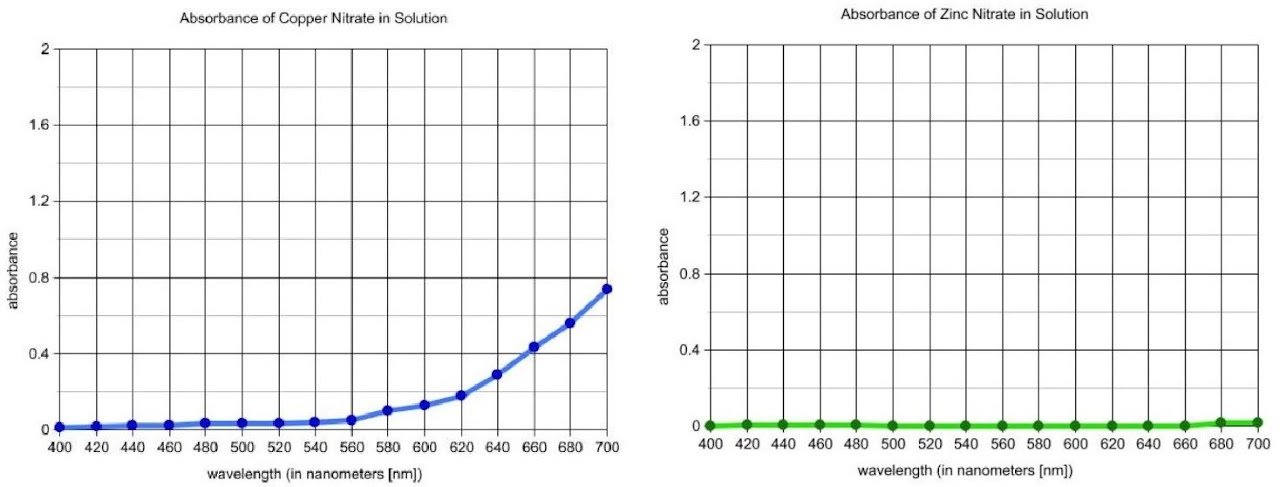
The purpose of this lab is to analyze the amount of copper in brass using visible spectroscopy. Brass can be dissolved by reacting it with concentrated nitric acid, which oxidizes the possible metal components of the alloy to their most common ions, Cu2+, Zn2+, and Fe3+. The lab begins with an introductory activity to distinguish among these metal ions using visible spectroscopy.
Students measure the absorbance of metal ion solutions at regular wavelength intervals from 400 nm to 700 nm and investigate the influence of the anion on the absorption spectra. The results provide a model for guided-inquiry design of an experiment to construct a calibration curve and determine the concentration of copper ions in a solution prepared by dissolving brass in nitric acid. Students must investigate the concentration range over which Beer's law is valid and identify the optimum wavelength for analysis.
Spectroscopy
This is a video that explains the basic concept on how a spectrophotometer works.
How a Spectrophotometer Works
A spectrophotometer measures the absorbance and transmittance of light through a sample. It operates as follows:
Light Source: A light source, such as a tungsten lamp (visible light) or a deuterium lamp (UV light), provides a continuous spectrum of light.
Monochromator: A monochromator (typically a diffraction grating or prism) selects a specific wavelength of light to pass through the sample.
Sample Holder: The selected light passes through a cuvette containing the sample solution. Cuvettes are made of glass, quartz (required for UV light), or plastic, depending on the wavelength range.
Detector: A detector (e.g., photodiode or photomultiplier tube) measures the intensity of light transmitted through the sample (I) and compares it to the intensity of the incident light (I₀) (measured without the sample).
Data Processing: The instrument calculates absorbance (A) using the equation:
A = -log(I/I₀)
Alternatively, transmittance (T) can be calculated as:
T = (I/I₀) × 100%
Output: The spectrophotometer displays absorbance or transmittance values. Absorbance is typically plotted against concentration (to create a calibration curve) or wavelength (to generate an absorption spectrum).