Physical Chemistry
People say “ugh” about organic chemistry … those people didn’t take PChem. Physical Chemistry focuses on the physical properties of matter and chemical reactions by applying the principles of physics, including concepts like thermodynamics, quantum mechanics, and kinetics to understand how molecules behave at the atomic and molecular level.
Virtual Tutor
More personalized support? Book a session tailored to exactly where you are stuck. At your own pace, real-time feedback, and most of all guidance for support beyond the session. Read more about our tutoring here.
We have tutors focused on helping students with just Physical Chemistry. Our goal is for you to be so incredibly happy, rich and that chemistry becomes so incredibly easy that your become world famous.
Group Sessions
CHEMDUNN hosts small groups of frustrated undergrads to unlock the most challenging concepts. It is like tutoring but done in small groups around a common topic. Think of them as seminars, workshops, mini-courses.
Learn more about groups here and check out our high school chemistry offerings here.
Ask a Tutor
You've spent time Googling. ChatGPT just isn't giving you the answer either (well maybe, but not sure). You just need a little help to get through that random problem which is blocking everything else.
Submit the question, get the answer and explanation to your inbox. Done. You can get full access to our content libraries and help from a human when the robots aren’t working or when you just need a person to interpret your incoherent cry for help. Get unstuck now.
Meet our Physical Chemistry Tutors
Lucia L.
Lead Tutor
(View bio)
Tutors are like public restroom hand dryers.
Some are the Dyson Airblades – powerful and get the job done quick (though a bit intense). Others are the standard blowers – they take a little longer, but eventually get you dry, kind of. But when you find that perfect dryer – the one with the right airflow and warmth that leaves you feeling completely dry – that's the tutor who really blows away the competition.
If you are not completely satisfied with your session you can switch to another tutor (free of charge) or get your money back. It is our Good Fit Guarantee.
Book a session here.
Semester 1
Physical Chemistry I
Thermodynamics
The Laws of Thermodynamics
Thermal equilibrium; Internal energy; Work and heat; Enthalpy and calorimetry; Entropy changes in reversible and irreversible processes; Spontaneity and the direction of processes; Carnot cycle and thermodynamic efficiency
Thermodynamic Properties of Systems
Ideal and real gases (van der Waals equation); Cv vs. Cp; Measuring heat changes; Enthalpies of formation and reaction; Bond enthalpies and lattice energy
Chemical Thermodynamics
ΔG and spontaneity; Relationship with equilibrium; Helmholtz Free Energy; Use in constant volume systems; Chemical Potential; Dependence on pressure and composition; Role in mixtures and phase transitions
Phase Equilibria
Phase Diagrams; Critical points and triple points; Clausius-Clapeyron Equation; Vapor pressure and boiling point; Chemical Potential in Phase Changes; Phase stability and transitions
Equilibrium Constants
Kp and Kc expressions; Relationship with ΔG; Le Châtelier’s Principle; Corrections for real systems
Electrochemistry
Galvanic and Electrolytic Cells; Cell diagrams and notation; Standard electrode potentials; Nernst Equation; Effect of concentration on potential; Thermodynamics of Electrochemical Cells; ΔG, ΔH, and ΔS from cell potentials
Chemical Kinetics
Differential and integrated rate laws; Half-life and pseudo-first-order reactions; Arrhenius equation; Activation energy; Elementary steps and rate-determining step; Steady-state and pre-equilibrium approximations
Surface and Catalysis
Adsorption Isotherms; Langmuir model; Catalysis; Homogeneous vs. heterogeneous; Reaction energy profiles
Semester 2
Physical Chemistry II
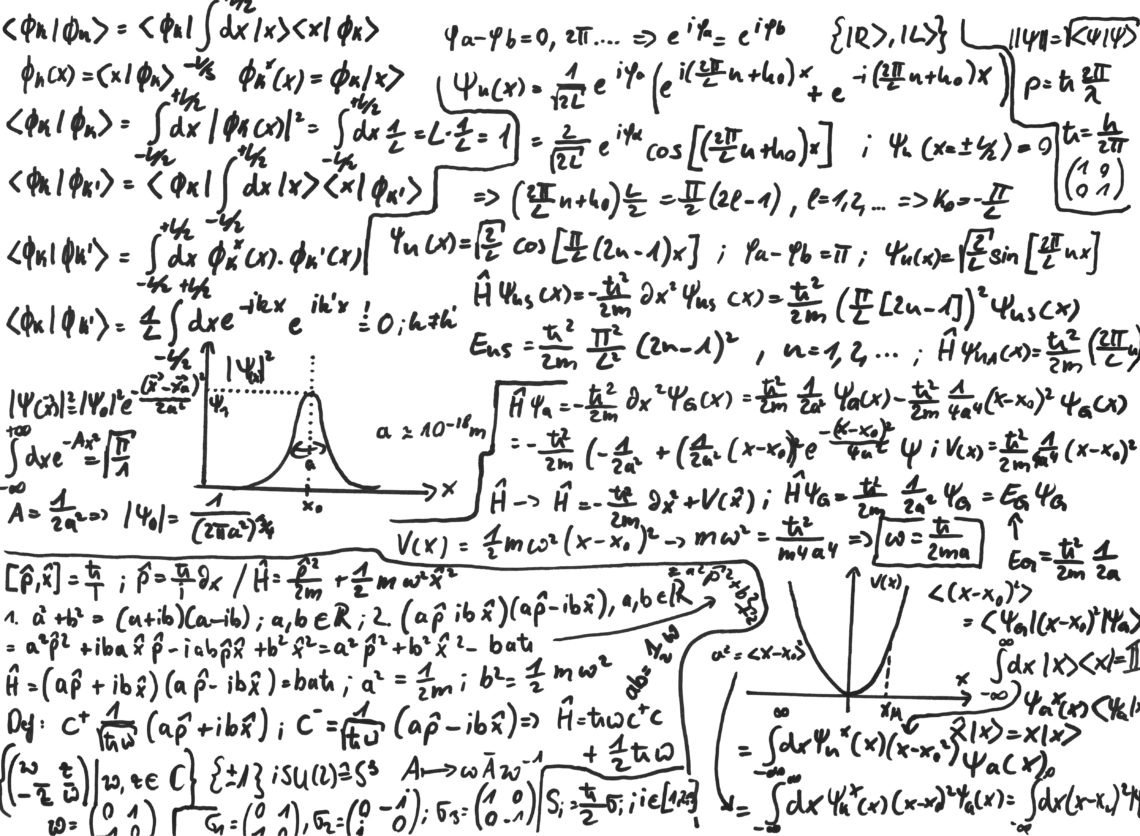
Quantum Mechanics
Conjugation and Dienes
Conjugated systems, resonance, and stability
Electrophilic addition to conjugated dienes
The Diels–Alder reaction (cycloaddition mechanism)
Aromatic Compounds and Reactivity
Structure and stability of benzene (aromaticity criteria)
Electrophilic aromatic substitution (EAS) mechanisms
Substituent effects (activating/deactivating groups, ortho/para/meta directors)
Nucleophilic aromatic substitution (if time allows)
Carbonyl Chemistry: Aldehydes and Ketones
Nomenclature, structure, and properties
Nucleophilic addition mechanisms (e.g., formation of hydrates, hemiacetals, acetals)
Oxidation and reduction (e.g., Tollens test, PCC, NaBH₄, LiAlH₄)
Imine and enamine formation
Carboxylic Acids and Their Derivatives
Nomenclature and properties of acids, esters, amides, acid chlorides, anhydrides
Nucleophilic acyl substitution reactions
Saponification, transesterification, and hydrolysis of amides
Interconversion of acid derivatives
Enolates and Enolate Reactions
Keto–enol tautomerism
Aldol condensation, crossed aldol reactions
Claisen condensation, Dieckmann condensation
Michael addition and Robinson annulation
Amines and Nitrogen-Containing Compounds
Nomenclature and basic properties of amines
Basicity, alkylation, and formation of amides
Reductive amination and other amine synthesis methods
Advanced Spectroscopy
More detailed ¹H NMR (complex splitting patterns, coupling constants)
¹³C NMR basics (chemical shifts, DEPT)
Advanced techniques (possibly 2D NMR, if time allows)
Expanded use of IR, UV-Vis, and Mass Spectrometry in structure elucidation
Carbohydrates, Amino Acids, and Other Biomolecules (Introduction)
Basic structures of monosaccharides and disaccharides (if covered)
Fischer and Haworth projections
Amino acid structure and properties; peptide bonds
Overview of lipids and nucleic acids (varies by course)
Synthesis Strategies
Multi-step synthesis combining reactions from both semesters
Retrosynthetic analysis (disconnection approach)
Protecting groups and strategic reaction pathways
Check out the other undergraduate courses that CHEMDUNN supports

