Intermolecular Forces
Related Examples and Practice Problems
Topic Related Question Bank Categories
Topic Summary & Highlights
and Help Videos
Core Concept
Intermolecular forces (IMFs) are forces of attraction or repulsion between molecules. These forces are weaker than intramolecular forces (bonds within a molecule), but they significantly impact the physical properties of substances such as boiling points, melting points, and solubility.
Purpose: IMFs determine the state of matter (solid, liquid, gas) and influence how molecules interact with each other in different phases.
Practice Tips
Memorize Common Ions: Focus on learning the common polyatomic ions, charges, and patterns.
Roman Numerals for Transition Metals: Practice associating transition metals with their possible charges.
Cross-Method for Formulas: To determine the correct formula, use the “criss-cross” method to balance charges between cations and anions.
Topic Overview Podcast
Topic Related Resources
|
LABORATORY
|
DEMONSTRATIONS
|
ACTIVITIES
|
VIRTUAL SIMULATIONS
|
There are three (3) primary types of Intermolecular Forces:
London Dispersion Forces (LDF)
Definition: London Dispersion Forces are the weakest intermolecular force, caused by temporary dipoles that occur when electrons in atoms or molecules shift positions momentarily.
Key Features:
Present in all molecules, whether polar or nonpolar.
Strength increases with the size and mass of the molecule.
Example: The interaction between noble gas atoms like helium (He) and neon (Ne) or between nonpolar molecules like oxygen (O₂) and nitrogen (N₂).
Factors Affecting LDF:
Molecular Size: Larger molecules with more electrons have stronger London dispersion forces.
Molecular Shape: Molecules with more surface area in contact with each other (linear vs. spherical shapes) experience stronger dispersion forces.
Dipole-Dipole Interactions
Definition: Dipole-dipole interactions occur between polar molecules, where positive and negative ends of permanent dipoles attract each other.
Key Features:
Present only in polar molecules, where there is a significant difference in electronegativity between atoms in a bond.
Stronger than London dispersion forces but weaker than hydrogen bonds.
Example: Interaction between molecules like hydrogen chloride (HCl) or sulfur dioxide (SO₂), where the dipoles align to maximize attractions between oppositely charged regions.
Factors Affecting Dipole-Dipole Interactions:
Molecular Polarity: The greater the dipole moment (difference in electronegativity between atoms), the stronger the dipole-dipole interaction.
Molecular Orientation: The alignment of dipoles influences the strength of interactions (stronger when aligned end-to-end).
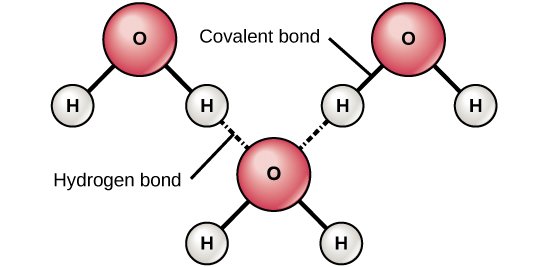
Hydrogen Bonding
Definition: Hydrogen bonding is a special type of dipole-dipole interaction that occurs when hydrogen is bonded to highly electronegative atoms such as nitrogen (N), oxygen (O), or fluorine (F). The partially positive hydrogen atom is attracted to the lone pair on a nearby electronegative atom.
Key Features:
Strongest type of intermolecular force among the three listed here.
Crucial in determining the structure and properties of water, DNA, and proteins.
Example: Water (H₂O) molecules form hydrogen bonds, which gives water its unique properties such as high boiling point and surface tension. Hydrogen bonds are also present in ammonia (NH₃) and hydrogen fluoride (HF).
Factors Affecting Hydrogen Bonding:
Electronegativity: The more electronegative the atom attached to hydrogen (N, O, or F), the stronger the hydrogen bond.
Molecular Geometry: The presence of lone pairs and the orientation of hydrogen bonds affect their strength.