Periodic Trends
Related Examples and Practice Problems
Topic Summary & Highlights
and Help Videos
Core Concept
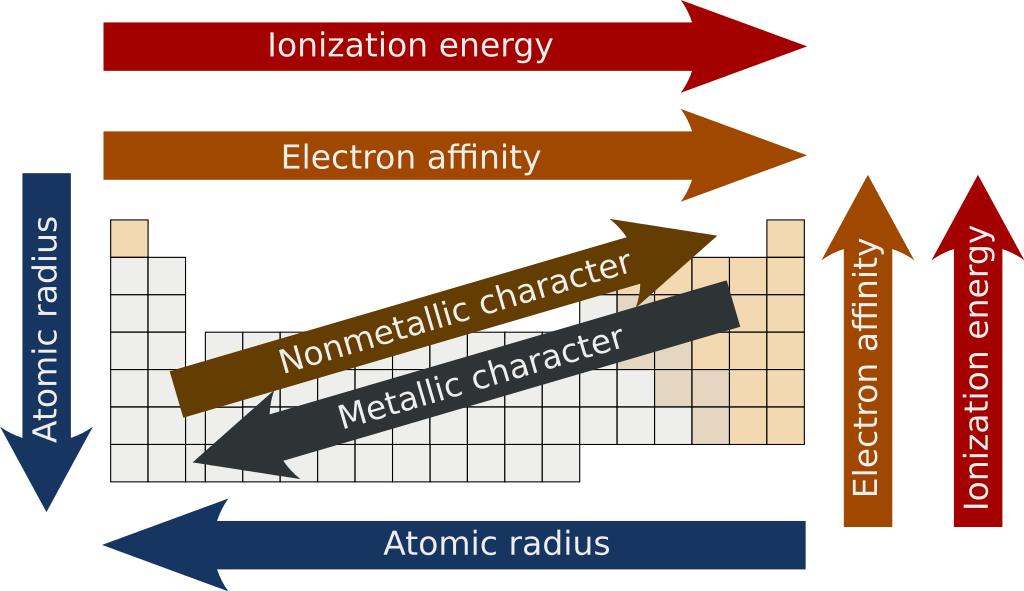
Atomic trends arise from the periodic table's structure and describe how properties like atomic radius, ionization energy, electronegativity, electron affinity, ion size, metallic character, and effective nuclear charge change across periods and down groups, influenced by the balance between nuclear charge, electron shielding, and energy levels.
Practice Tips
Use the periodic table as a visual aid to see where each trend increases or decreases.
Practice predicting properties of unknown elements based on their position relative to known elements.
Understand the "why" for each trend—focus on how nuclear charge and electron shells influence each trend.
Practice with examples: Compare the properties of elements in the same group and period, like comparing sodium (Na) and chlorine (Cl) for trends across a period, or lithium (Li) and potassium (K) for trends down a group.
Topic Overview Podcast
Topic Related Resources
|
LABORATORY
|
DEMONSTRATIONS
|
ACTIVITIES
|
VIRTUAL SIMULATIONS
|
Atomic Radius
Definition: The distance from the nucleus to the outermost electron shell.
Trend:
Across a Period (Left to Right): Atomic radius decreases.
Why? Increased nuclear charge (more protons) pulls electrons closer to the nucleus without adding additional electron shells.
Down a Group (Top to Bottom): Atomic radius increases.
Why? Each row down adds a new electron shell, increasing the size of the atom.
Electron Affinity (EA)
Definition: The energy change that occurs when an atom gains an electron, typically resulting in a negative value (energy is released).
Trend:
Across a Period: Electron affinity becomes more negative (favorable) in general.
Why? Atoms on the right side of the periodic table (nonmetals) have a stronger tendency to gain electrons to complete their valence shells.
Down a Group: Electron affinity becomes less negative.
Why? Larger atomic size reduces the attraction between the nucleus and added electrons.
Note: Noble gases do not follow the trend as they have full valence shells and generally do not gain electrons.
Ionization Energy
Definition: The energy required to remove an electron from an atom in its gaseous state.
Trend:
Across a Period: Ionization energy generally increases.
Why? As atomic radius decreases, electrons are closer to the nucleus and more tightly held, requiring more energy to remove.
Down a Group: Ionization energy decreases.
Why? Electrons are further from the nucleus and experience less attraction, making them easier to remove.
Exceptions: There are slight decreases in ionization energy within periods due to electron repulsion in p-orbitals and half-filled orbital stability.
Electronegativity
Definition: A measure of an atom's ability to attract and bond with electrons when in a compound.
Trend:
Across a Period: Electronegativity increases.
Why? Nonmetals on the right side need only a few electrons to achieve a stable electron configuration, so they attract electrons more strongly.
Down a Group: Electronegativity decreases.
Why? Increased atomic size reduces the pull of the nucleus on bonding electrons.
Effective Nuclear Charge (Zeff)
CHECK OUT THE EFFECTIVE NUCLEAR CHARGE TOPIC PAGE HERE.
Definition: The net positive charge experienced by valence electrons after accounting for shielding from inner electrons.
Trend:
Across a Period: Z_eff increases.
Why? With each additional proton, the nuclear charge increases, but added electrons are in the same shell and don’t shield each other effectively.
Down a Group: Z_eff slightly decreases or stays roughly constant.
Why? New shells reduce the nuclear pull on outer electrons despite the increase in nuclear charge.
Ionic Size
Definition: The tendency of an element to lose electrons and form cations (typical of metals).
When an atom (neutral) becomes an ion (charged) it either loses or gains electrons.
Atoms (trend to be metals) that LOSE electrons become CATIONS (positively charged). Atoms get SMALLER when an electron is LOST.
Why? xx
Atoms (tend to be non-metals) that GAIN electrons become ANIONS (negatively charged). Atoms get LARGER when an electron is GAINED.
Why? xx
| Property | Across a Period | Down a Group |
|---|---|---|
| Atomic Radius | Decreases | Increases |
| Ionization Energy | Increases | Decreases |
| Electronegativity | Increases | Decreases |
| Electron Affinity | Becomes more negative | Becomes less negative |
| Ion Size | Decreases (for same charge) | Increases |
| Metallic Character | Decreases | Increases |
| Effective Nuclear Charge (\( Z_{\text{eff}} \)) | Increases | Remains relatively constant |
Metallic Character
Definition: The tendency of an element to lose electrons and form cations (typical of metals).
Trend:
Across a Period: Metallic character decreases.
Why? Moving across a period, elements more readily gain electrons rather than lose them, as they approach a full valence shell.
Down a Group: Metallic character increases.
Why? Larger atoms with lower ionization energies more easily lose electrons, a characteristic of metals.