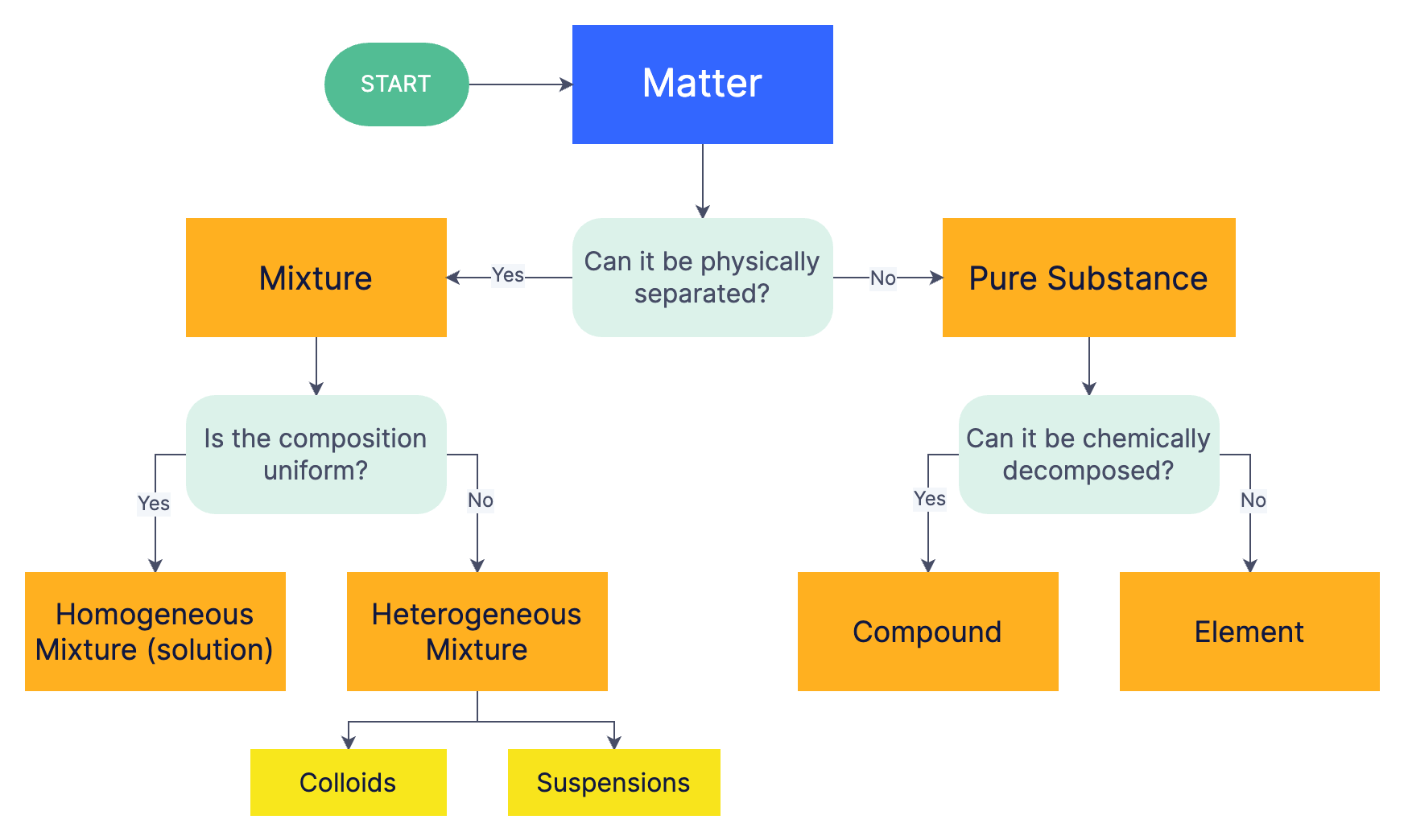
Classification of Matter
Related Examples and Practice Problems
Topic Summary & Highlights
and Help Videos
Core Concept
Matter is classified based on its composition into pure substances (elements and compounds) and mixtures (homogeneous and heterogeneous). Pure substances consist of only one type of particle, such as atoms in elements or molecules in compounds, while mixtures contain two or more substances physically combined but not chemically bonded. Particles themselves are classified into atoms, ions, and molecules, with atoms being the smallest units of elements and molecules formed by bonded atoms.
Study Tips
Classification of Matter: Matter is divided into pure substances (elements and compounds) and mixtures (homogeneous and heterogeneous).
Particles of Matter: Atoms are basic units, molecules are bonded atoms, and ions are charged particles.
Distinctions: Compounds require chemical methods to separate, while mixtures use physical methods.
Separation Techniques: Mixtures are separated using filtration, distillation, or chromatography.
Core Concept
States of matter are the different physical forms in which matter can exist. Matter can exist as a solid, liquid, or gas, depending on the arrangement and movement of its particles.
Solids have a fixed shape and volume. The particles in a solid are tightly packed and held together by strong intermolecular forces, resulting in a rigid structure. They vibrate in place but do not move freely.
Important properties often emphasized with solids: density, hardness, and melting point.
Liquids have a definite volume but no fixed shape. The particles in a liquid are close together, but they have more freedom of movement compared to solids. They can slide past each other, allowing liquids to flow and take the shape of their containers.
Important properties often emphasized with liquids: viscosity, surface tension, and boiling point.
Gases have neither a fixed shape nor volume. The particles in a gas are far apart and move randomly and rapidly. They have high kinetic energy (move fast), enabling them to occupy the entire volume of their container and exert pressure on its walls.
Important properties often emphasized with gases: compressibility, pressure, and temperature.
Important Terms Defined:
Mixture: A physical combination of two or more substances that retain their individual chemical identities. Think of trail mix.
Pure substance: A form of matter with a fixed composition and consistent properties throughout. It can be either an element or a compound. Imagine a pure gold bar or sugar.
Homogeneous mixture: A uniform mixture where the components are evenly distributed. You can't visually distinguish different parts. Picture well-stirred lemonade - the sugar and water are evenly mixed.
Heterogeneous mixture: A non-uniform mixture where the components are visibly distinguishable. Think concrete.
Compound: A pure substance formed by the chemical combination of two or more different elements in a fixed ratio such as water (H₂O) .
Element: A pure substance that cannot be broken down into simpler substances by chemical means. It consists of only one type of atom.
Colloids: Homogeneous mixtures with particle sizes ranging from 1-100 nanometers. These particles are too small to settle out but large enough to scatter light, giving the mixture a cloudy appearance. Milk is a colloid.
Suspensions: Heterogeneous mixtures where larger particles (greater than 100 nanometers) are dispersed throughout a liquid and tend to settle out over time if left undisturbed. Muddy water is a suspension.