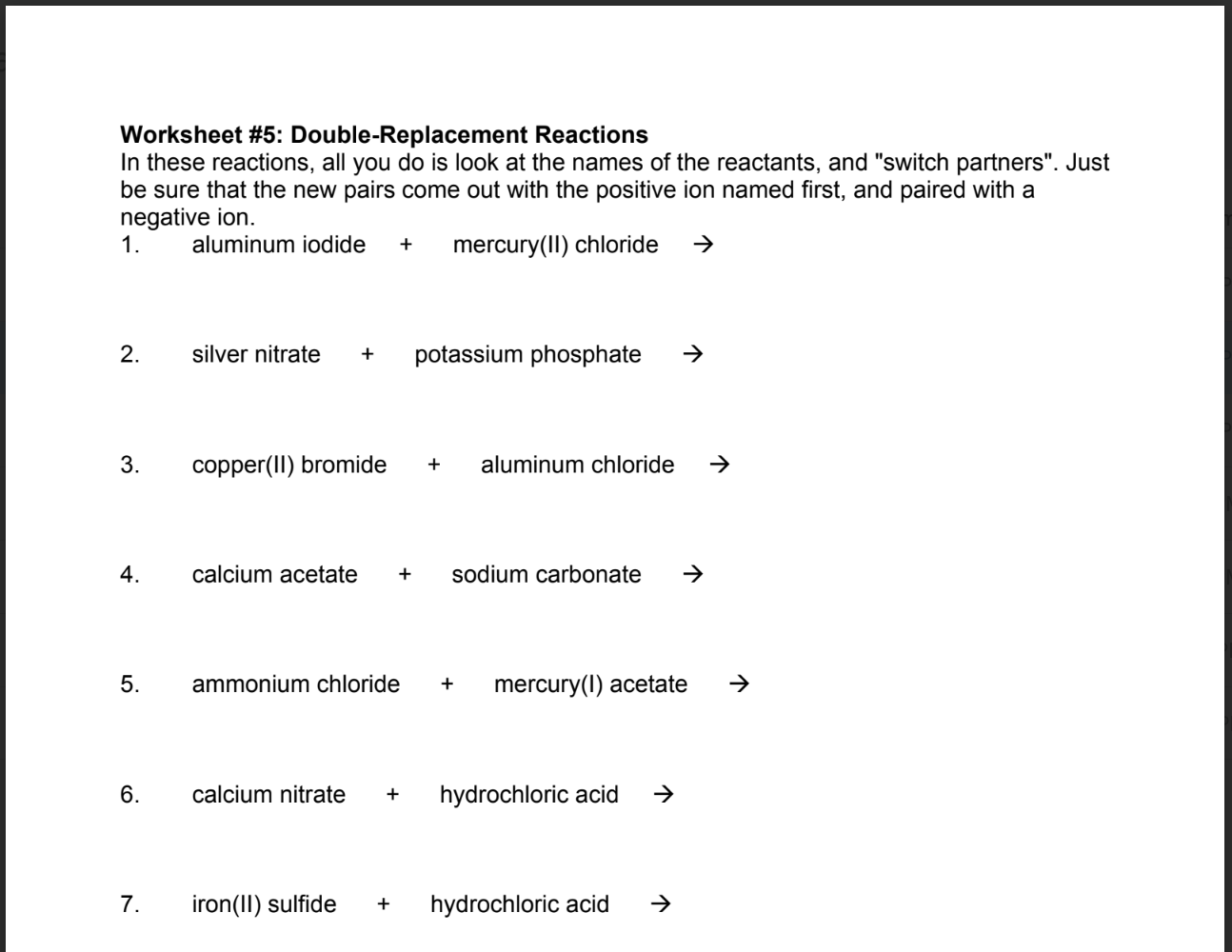
Double Displacement
Related Examples and Practice Problems
Additional Worked Out Examples/ Practice
Identifying classification types: Differentiation between elements, compounds or mixtures and homogeneous and heterogenous mixtures
Separation techniques: Selected and explaining limitation of appropriate separation
Relating Properties to Composition: Predicting classification based on descriptive properties
Topic Summary & Highlights
and Help Videos
Core Concept
Double displacement reactions involve the exchange of ions between two compounds in solution, resulting in the formation of two new compounds. These reactions follow the general form: AB+CD→AD+CB
Where:
AB and CD are ionic compounds.
The positive ion (cation) of the first compound (A) swaps with the positive ion of the second compound (C).
Practice Tips
Use Solubility Rules: These help you determine if a precipitate forms and if the reaction will proceed.
Balance Carefully: Ensure the equation has the same number of atoms on both sides.
Recognize Patterns: Precipitation, neutralization, and gas formation are common indicators of double displacement reactions.
Identify Non-Reactive Cases: If all products are soluble and no gas or water forms, no reaction occurs.
Types of Double Displacement Reactions
Precipitation Reactions: A solid (precipitate) forms when two aqueous solutions of ionic compounds are mixed.
Acid-Base Neutralization Reactions: An acid reacts with a base to produce a salt and water.
Gas-Forming Reactions: A gas is produced when certain compounds react, such as when an acid reacts with a carbonate.
Predicting Double Displacement Reactions
To determine if a double displacement reaction will occur, you need to consider the solubility rules and formation of specific products like precipitates, water, or gas.
Solubility Rules
Always Soluble:
Compounds containing alkali metal ions (Li⁺, Na⁺, K⁺) and ammonium (NH₄⁺).
Nitrates (NO₃⁻), acetates (CH₃COO⁻), and perchlorates (ClO₄⁻) are generally soluble.
Insoluble Compounds (usually form precipitates):
Carbonates (CO₃²⁻), phosphates (PO₄³⁻), and sulfides (S²⁻), unless with alkali metals or ammonium.
Halides (Cl⁻, Br⁻, I⁻) are generally soluble except with Ag⁺, Pb²⁺, and Hg₂²⁺.
Sulfates (SO₄²⁻) are soluble, except with Ba²⁺, Sr²⁺, and Pb²⁺.
Steps to Predict and Write Double Displacement Reactions
Write the Reactants and Swap the Ions:
Identify the cations and anions in each reactant.
Swap the anions between the two compounds.
Write the Products:
Combine each cation with the opposite anion to form the products.
Be sure to write correct formulas based on the charges of ions.
Check Solubility:
Use the solubility rules to determine if any of the products are insoluble (forming a precipitate).
If a precipitate, gas, or water forms, the reaction will likely occur.
Balance the Equation:
Adjust coefficients to balance the atoms on both sides of the equation.
Example Problems
Example 1: Precipitation Reaction
Problem: Will a reaction occur if solutions of barium chloride (BaCl2\text{BaCl}_2BaCl2) and sodium sulfate (Na2SO4\text{Na}_2\text{SO}_4Na2SO4) are mixed? Write the balanced equation.
Write the Reactants and Swap Ions:
Ba2+\text{Ba}^{2+}Ba2+ and Cl−\text{Cl}^-Cl−; Na+\text{Na}^+Na+ and SO42−\text{SO}_4^{2-}SO42−.
Products: BaSO4\text{BaSO}_4BaSO4 and NaCl\text{NaCl}NaCl.
Check Solubility:
BaSO4\text{BaSO}_4BaSO4 is insoluble (forms a precipitate).
NaCl\text{NaCl}NaCl is soluble.
Write and Balance the Equation:
BaCl2(aq)+Na2SO4(aq)→BaSO4(s)+2NaCl(aq)\text{BaCl}_2 \text{(aq)} + \text{Na}_2\text{SO}_4 \text{(aq)} \rightarrow \text{BaSO}_4 \text{(s)} + 2\text{NaCl} \text{(aq)}BaCl2(aq)+Na2SO4(aq)→BaSO4(s)+2NaCl(aq)
Answer: Yes, a reaction occurs, and a precipitate of BaSO4\text{BaSO}_4BaSO4 forms.
Example 2: Acid-Base Neutralization
Problem: Predict the products and balance the equation for the reaction between hydrochloric acid (HCl\text{HCl}HCl) and potassium hydroxide (KOH\text{KOH}KOH).
Write the Reactants and Swap Ions:
H+\text{H}^+H+ and Cl−\text{Cl}^-Cl−; K+\text{K}^+K+ and OH−\text{OH}^-OH−.
Products: KCl\text{KCl}KCl and H2O\text{H}_2\text{O}H2O.
Check for Solubility:
Both KCl\text{KCl}KCl is soluble, and H2O\text{H}_2\text{O}H2O is a stable liquid.
Write and Balance the Equation:
HCl(aq)+KOH(aq)→KCl(aq)+H2O(l)\text{HCl} \text{(aq)} + \text{KOH} \text{(aq)} \rightarrow \text{KCl} \text{(aq)} + \text{H}_2\text{O} \text{(l)}HCl(aq)+KOH(aq)→KCl(aq)+H2O(l)
Answer: A neutralization reaction occurs, producing KCl\text{KCl}KCl and water.
Example 3: Gas-Forming Reaction
Problem: Predict the products and balance the equation for the reaction between calcium carbonate (CaCO3\text{CaCO}_3CaCO3) and hydrochloric acid (HCl\text{HCl}HCl).
Write the Reactants and Swap Ions:
Ca2+\text{Ca}^{2+}Ca2+ and CO32−\text{CO}_3^{2-}CO32−; H+\text{H}^+H+ and Cl−\text{Cl}^-Cl−.
Products: CaCl2\text{CaCl}_2CaCl2 and H2CO3\text{H}_2\text{CO}_3H2CO3.
Decompose the Unstable Product:
H2CO3\text{H}_2\text{CO}_3H2CO3 is unstable and decomposes into CO2\text{CO}_2CO2 gas and H2O\text{H}_2\text{O}H2O.
Write and Balance the Equation:
CaCO3(s)+2HCl(aq)→CaCl2(aq)+CO2(g)+H2O(l)\text{CaCO}_3 \text{(s)} + 2\text{HCl} \text{(aq)} \rightarrow \text{CaCl}_2 \text{(aq)} + \text{CO}_2 \text{(g)} + \text{H}_2\text{O} \text{(l)}CaCO3(s)+2HCl(aq)→CaCl2(aq)+CO2(g)+H2O(l)
Answer: A gas-forming reaction occurs, producing calcium chloride, carbon dioxide, and water.