Paramagnetism and Diamagnetism
Related Examples and Practice Problems
Topic Summary & Highlights
and Help Videos
Core Concept
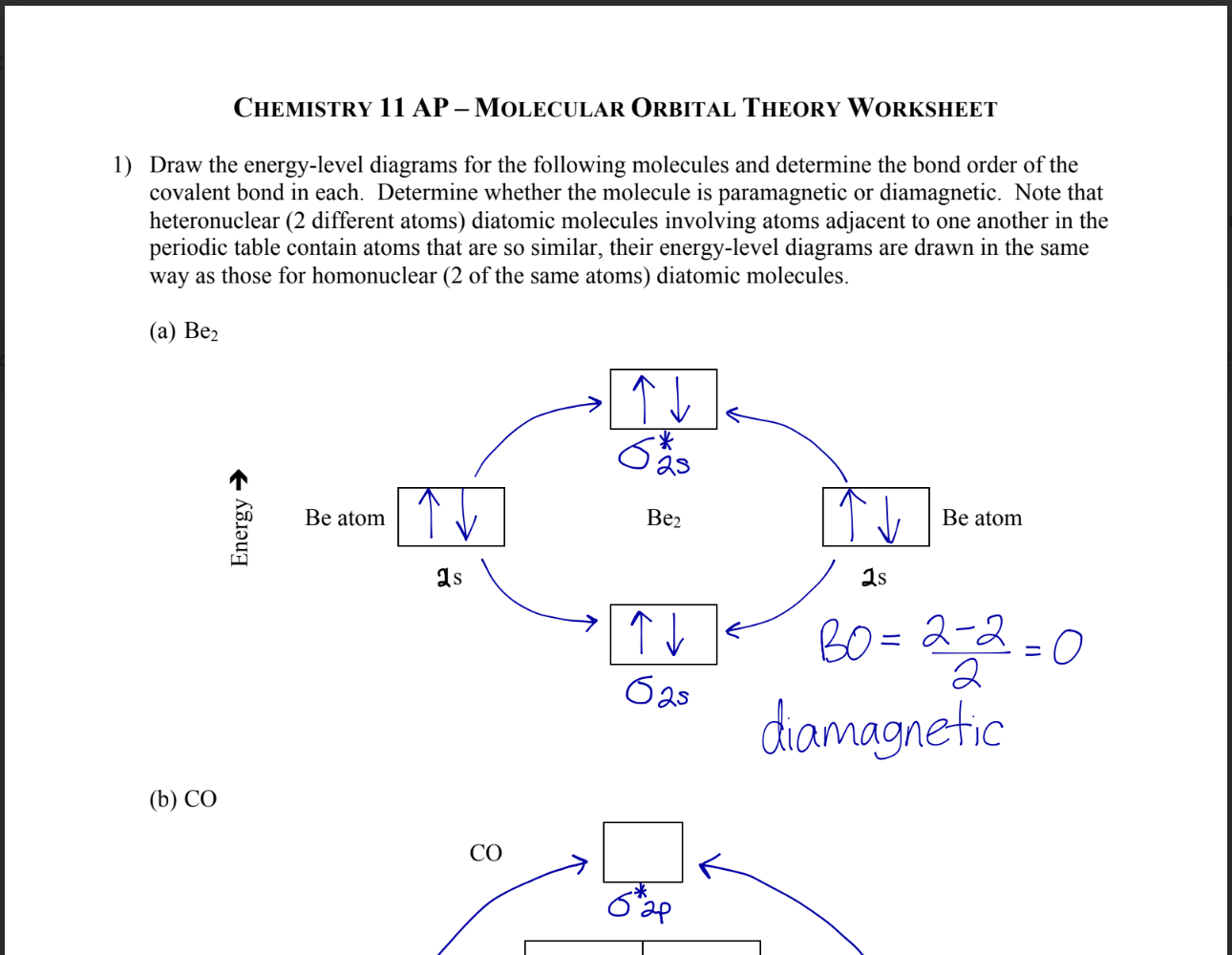
Paramagnetic substances have unpaired electrons, which align with external magnetic fields, making the substance attracted to the field, while diamagnetic substances have all electrons paired, causing them to create an opposing magnetic field and be weakly repelled by external magnetic fields. These properties arise from the electron configurations of atoms or ions.
Practice Tips
Use diagrams to visualize the electron spins and their alignment in paramagnetic and diamagnetic materials.
Relate paramagnetism and diamagnetism to electron configurations of elements.
Look for periodic trends in magnetic behavior across different groups of elements.
Topic Overview Podcast
Topic Related Resources
|
LABORATORY
|
DEMONSTRATIONS
|
ACTIVITIES
|
VIRTUAL SIMULATIONS
|
Definitions
Paramagnetism: Imagine tiny atomic bar magnets. In paramagnetic materials, some electrons have unpaired spins (ms = +1/2 or -1/2). These unpaired spins act like miniature magnets, and when a magnetic field is applied, they tend to align with the field. This slight alignment creates a weak overall attraction, making the material weakly paramagnetic.
Key features:
Elements with unpaired electrons are likely to be paramagnetic.
Transition metals often exhibit paramagnetism due to unpaired electrons in their d orbitals.
The strength of paramagnetism depends on the number of unpaired electrons and the orbital they occupy.
Diamagnetism: On the other hand, diamagnetic materials have all their electrons paired (each with opposite spins). In a diamagnetic atom, the circulating electrons create tiny magnetic fields that oppose any external magnetic field. This opposition results in a weak repulsion force, making the material slightly diamagnetic.
Key features:
Elements with all electrons paired are likely to be diamagnetic.
Noble gas elements (Group 18) are classic examples of diamagnetic elements.
Diamagnetism is a weaker effect compared to paramagnetism.
Distinguishing Between the Two:
Experimental Technique: Scientists use a technique called magnetic susceptibility to measure how a material responds to a magnetic field. A positive susceptibility indicates paramagnetism, while a negative susceptibility suggests diamagnetism.
Beyond the Basics:
Ferromagnetism: This is a much stronger form of magnetism where the electron spins in a material are aligned even in the absence of an external field, creating a permanent magnet.
Antiferromagnetism: In some materials, neighboring electron spins are anti-aligned, cancelling out their magnetic effects, resulting in a material with no net magnetic moment.