Single Replacement
Related Examples and Practice Problems
Additional Worked Out Examples/ Practice
Identifying classification types: Differentiation between elements, compounds or mixtures and homogeneous and heterogenous mixtures
Separation techniques: Selected and explaining limitation of appropriate separation
Relating Properties to Composition: Predicting classification based on descriptive properties
Topic Summary & Highlights
and Help Videos
Core Concept
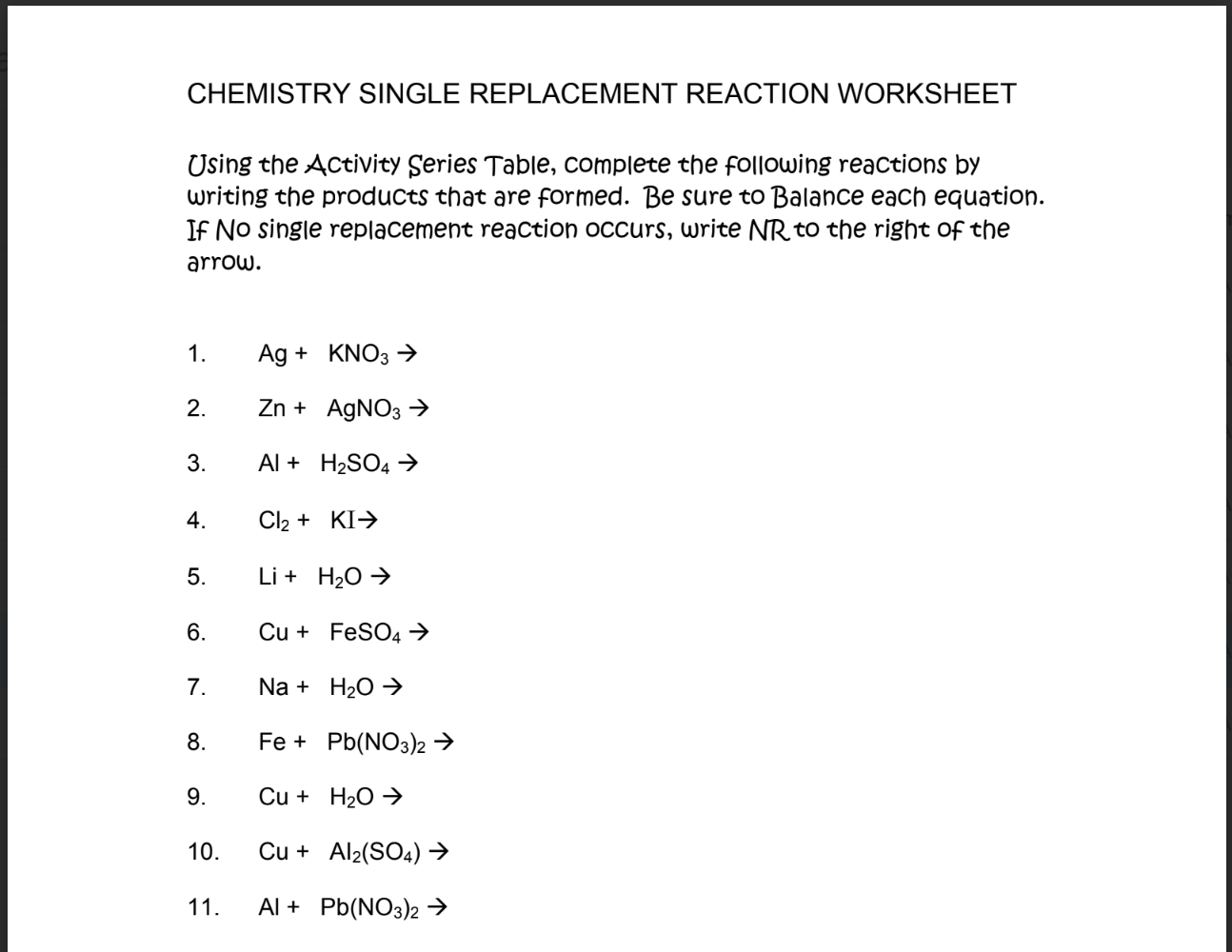
Single replacement reactions (also called single displacement reactions) involve one element replacing another in a compound. These reactions follow the general form: A+BC→AC+B
Where:
A is a single element.
BC is a compound.
A replaces B in BC if A is more reactive than B.
Practice Tips
Memorize Common Ions: Focus on learning the common polyatomic ions, charges, and patterns.
Roman Numerals for Transition Metals: Practice associating transition metals with their possible charges.
Cross-Method for Formulas: To determine the correct formula, use the “criss-cross” method to balance charges between cations and anions.
Topic Overview Podcast
Topic Related Resources
|
LABORATORY
|
DEMONSTRATIONS
|
ACTIVITIES
|
VIRTUAL SIMULATIONS
|
Core Concept
Single replacement reactions (also called single displacement reactions) involve one element replacing another in a compound. These reactions follow the general form:
A+BC→AC+B
Where:
A is a single element.
BC is a compound.
A replaces B in BC if A is more reactive than B.
Types of Single Replacement Reactions
Metal Replacing Metal:
A metal in its elemental form replaces another metal in a compound.
Example: Zn+CuSO4→ZnSO4+Cu\text{Zn} + \text{CuSO}_4 \rightarrow \text{ZnSO}_4 + \text{Cu}Zn+CuSO4→ZnSO4+Cu
Metal Replacing Hydrogen:
A metal can replace hydrogen in acids or water (e.g., alkali metals reacting with water).
Example: Mg+2HCl→MgCl2+H2\text{Mg} + 2\text{HCl} \rightarrow \text{MgCl}_2 + \text{H}_2Mg+2HCl→MgCl2+H2
Halogen Replacing Halogen:
A halogen in its elemental form replaces another halogen in a compound.
Example: Cl2+2KI→2KCl+I2\text{Cl}_2 + 2\text{KI} \rightarrow 2\text{KCl} + \text{I}_2Cl2+2KI→2KCl+I2
The Activity Series
The activity series is a list of metals and halogens ranked by their reactivity. In single replacement reactions, an element can replace another element in a compound only if it is higher on the activity series.
Key Points:
More reactive elements are at the top of the activity series.
Less reactive elements are at the bottom.
Metals at the top of the series (e.g., lithium, potassium) are highly reactive and can replace metals lower in the series.
Halogens also follow an activity trend, with fluorine being the most reactive.
Predicting Reactions Using the Activity Series
Identify the Elements Involved:
Look at the single element (A) and the element it might replace in the compound (B).
Check the Activity Series:
Find both elements in the activity series.
If A is higher on the activity series than B, the reaction will occur, and A will replace B.
Write the Products:
Replace B with A in the compound to predict the products.
If A is Lower:
If A is lower on the activity series than B, no reaction will occur.